Trinitite is an artificial "mineral" (also known as "Atomic Bomb Glass") created during the first ever nuclear explosion test in the desert of New Mexico - "The Trinity Test" on July 16, 1945. During the nuclear explosion, desert sand was sucked into the fireball, melted while being infused with fission products and deposited back on the desert floor as "glassy" rocks.
The "classic" Trinitite has olive-greenish, (often smooth on the top and rough on the bottom) . glassy surface with many irregular inclusions and gas pockets - technically, the nuclear explosion created glass out of the desert sand by melting it while including radionuclides and droplets of iron and copper from the supporting tower and wiring. The structure and make of this man-made mineral is complex and diverse, based on the exact location in the blast zone where it came from.
After the end of the war, when the Manhattan project was revealed to the public, people started collecting Trinitite from the White Sands Missile Range. Once the US Government and Army caught a wind of this, in 1954 they forbade any collection of samples from Ground Zero and made this activity illegal. The Trinity site is also off-limits to the public except for one day in the year with a guided tour, so real Trinitite has somewhat limited availability and it can be difficult to find / buy nowadays, thus becoming somewhat expensive.
My sample didn't look exactly as what I've seen on pictures of typical Trinitite but it was showing an activity of around 500-550 CPM on my LND7317 "pancake" detector equipped GQ GMC-600+ Geiger Counter.
My sample didn't look exactly as what I've seen on pictures of typical Trinitite but it was showing an activity of around 500-550 CPM on my LND7317 "pancake" detector equipped GQ GMC-600+ Geiger Counter.
Mildly radioactive rocks can be passed as "Trinitite" especially the ones with greenish tint (minerals with micro-crystals of Torbernite on the surface can somewhat resemble the "Trinitite look").
I was wondering if I got a "fake Trinitite" - Uranium or Thorium mineral, showing natural radioactivity or it was the "real deal" - it was a "free gift" anyways but it did spark my curiosity and I didn't have any other actual Trinitite in my collection. To be honest, I was a bit skeptical about it at first and thought it just might be an unidentified radioactive mineral.
Upon a close examination, a promising feature of my sample was notable - the extremely porous nature in cross-cut with many gas pockets but relatively smooth on one surface - a result from Trinitite being cooked by a giant gaseous fireball overhead - the hot gases from the explosion melting and mixing the desert sand yield a porous, almost Vulcanic / magma looking rock.
Upon a close examination, a promising feature of my sample was notable - the extremely porous nature in cross-cut with many gas pockets but relatively smooth on one surface - a result from Trinitite being cooked by a giant gaseous fireball overhead - the hot gases from the explosion melting and mixing the desert sand yield a porous, almost Vulcanic / magma looking rock.
The somewhat coarse and "frothy" surface is due to the shockwave of the blast, rolling pieces of melted sand across the desert floor - another evidence that it came from an area close to the epicenter of the explosion is the higher level of radioactivity. Most of the trinitite samples in circulation will have one side (the top side) smooth and glassy while the opposite side will be rough and coarse.
The Trinitite specimen loaded into the lead-shielded sample chamber of my "Gamma-nator 2000" 😀 Gamma Spectrometer.
The sample is placed in a plastic bag in order to prevent contamination of the test chamber and then placed in a copper sample holder.
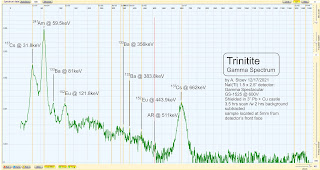
Theremino MCA displaying the Gamma Spectrum Analysis of my Trinitite specimen (22 hr. scan).
A 22 hours spectrum scan with the background (22 hours scan as well) subtracted.
The sample is located <1cm from the front of the detector.
The desert sand in New Mexico has traces or Eu-151 but the only way to get Eu-152 (which is not a naturally occurring isotope) is by neutron activation and the neutron capture of Eu-151 . Caesium-137 and Americium-241 are also not naturally occurring isotopes. Americium-241 is created by the neutron bombardment of Pu-239 + 2(n) -> Pu-241 -> Am-241. Cs-137 is an infamous fission by-product of splitting Uranium and Plutonium nuclei.
While Trinity was a Plutonium-based device, natural Uranium was used as tamper/pusher and lots of it too, which also explains the U-235 peak.
This gamma spectrum is the typical gamma "fingerprint" of Trinitite - none of the observed activity is natural other than the small K-40 peak.
For a reference, this mineral from my collection - Meta-Zeunerite (Hydrated Copper-Uranium Arsenate - Cu(UO₂)₂(AsO₄)₂•H₂O) is a secondary Uranium mineral with natural radioactivity due to U-238, U-235 and a slew of daughter products.
This gamma spectrum is the typical gamma "fingerprint" of Trinitite - none of the observed activity is natural other than the small K-40 peak.
In conclusion - this specimen indeed is a piece of history and it was born under a giant Mushroom Cloud at the White Sands Missile Range, NM.
I also purchased a sample of Trinitite from Scott over at the Atomic Rock Shop (www.atomicrockshop.com). This is a great resource for anyone who wants to own a piece of history and the buying experience was excellent.
This sample exhibits the "typical" Trinitite look - much deeper olive-green color and very glassy-smooth surface. As suspected the activity is lower as this piece of Trinitite likely came from an area further away from the blast where the sand was just melted and cooled down with less disturbance.
No surprises in the spectrum (a 4.5 hrs scan with Gamma Spectacular GS-1525 NaI(Tl) detector) - the typical isotopic mixture of Cs-137, Am-241 and Eu-152. The amount of Europium-152 in this sample appears to be less than the first sample, again consistent with a greater distance to the epicenter and lower neutron flux.
The Geiger Counter activity measures approximately 170-200 CPM @ 5 mm from the surface (Alpha-particles shielded by plastic) using an LND 7317 tube, so it is quite safe to keep in a display box on your desk or shelf.
This particular specimen is from the Clara Mine, Oberwolfach, Ortenaukreis, Freiburg Region, Baden-Württemberg, Germany. Activity is around 300 CPM.
Here is the Gamma-Spectrum of the Zeunerite sample- a classic natural Uranium spectrum with a number of daughter product peaks - Lead-214, Bismuth-214, Ra-226 etc.
Update:
Michael Magiera of Trinitite Rocks asked me to do another Trinitite scan of a sample he provided and this time I didn't go for the typical 6-8 hrs. Sometimes small peaks are lost during long scan and especially if smoothing/flitering is used. I scanned the sample only 3.5 hours (/w 2 hours of background subtraction) and this scan revealed something that I have seen before in Trinitite samples but not really paying too much attention at it...
This spectrum clearly reveals Ba-133 photopeaks! This begs the question - where this Barium is coming from? Initially it made no sense to see Ba-133 and I was ready to chalk it off as an environmental influence or something parasitic in the detector. Then I looked deeper into the design of the Gadget.
The explosive lenses used to implode the Pu core of the "Gadget" were made out of fast and slow explosives in order to control the shockwave and achieve an equally distributed and uniform implosion force - this was the whole purpose of the "lens" assembly. The geometry and explosive compounds used for different parts act as shape-charges creating multiple shockwaves with different velocities, canceling peaks and valleys in the shockwave until all eventually evens out at the surface of the core squeezing it uniformly from all sides.
As it turns out, for the "slow" explosive components in the lenses, they used "Baratol" - a lower velocity explosive compound which is made of a mixture of TNT and Barium Nitrate (nearly 70% of the mixture). The Barium Nitrate contains natural, stable isotopes of Ba-130 and Ba-132 which, when exposed to the incredible neutron flux undergo neutron capture and are converted into the synthetic Ba-133 isotope seen in this spectrum.
It is pretty amazing to realize that as the bomb exploded, the intense neutron flux even converted the conventional explosives into radioactive nuclides and I can detect this at home, almost 80 years later (or nearly 8 half-lives of Ba-133 (10.55y)) in a piece of melted sand with the spectroscopy setup I built.
















2 comments:
Under your Update section, the test you performed was on my Trinitite. It would be a great help if you could mention Trinitite Rocks as the source instead of "Someone". Currently I began an Etsy Shop while my website is being created. Thank you, Michael Magiera
Edited the post to reflect the source of the sample.
Post a Comment